Who is Involved with a Clinical Trial

Principle Investigator (PI): The PIs are osteopathic physicians who are responsible for leading the clinical trial. They oversee all aspects of the study, ensuring proper execution, and serve as physicians to the participants.
Osteopathic Fellows (OMM Fellows): OMM Fellows are osteopathic medical students participating in the research team. Their responsibilities include recruiting participants, collecting data, and assisting with OMT, gaining hands-on experience under supervision.
Clinical Research Coordinator (CRC): The CRC acts as the central point of contact for all clinical trial activities. They manage participant recruitment and data collection and ensure that the study follows all protocols and regulatory requirements. They communicate with the IRB and update all researchers on the study's progress. They will work most closely with you throughout the study.
Institutional Review Board (IRB): The IRB is a committee that reviews all research proposals to safeguard participant safety, data integrity, and ethical standards. While you may not interact directly with them, the IRB plays a critical role in protecting your rights as a participant. Regular reports are submitted to the IRB to ensure ongoing compliance throughout the study.
Participants: You, the participants, are the most important part of the clinical trial. By volunteering and meeting the study’s criteria, you make these research efforts possible. Your contribution is invaluable to advancing osteopathic medicine and improving future treatments.
Reminder to Potential Participants
As you consider joining a clinical trial, it's important to remember that your participation is always voluntary. Even if you decide to enroll, you have the right to withdraw from the trial at any time and for any reason—your choice is always respected.
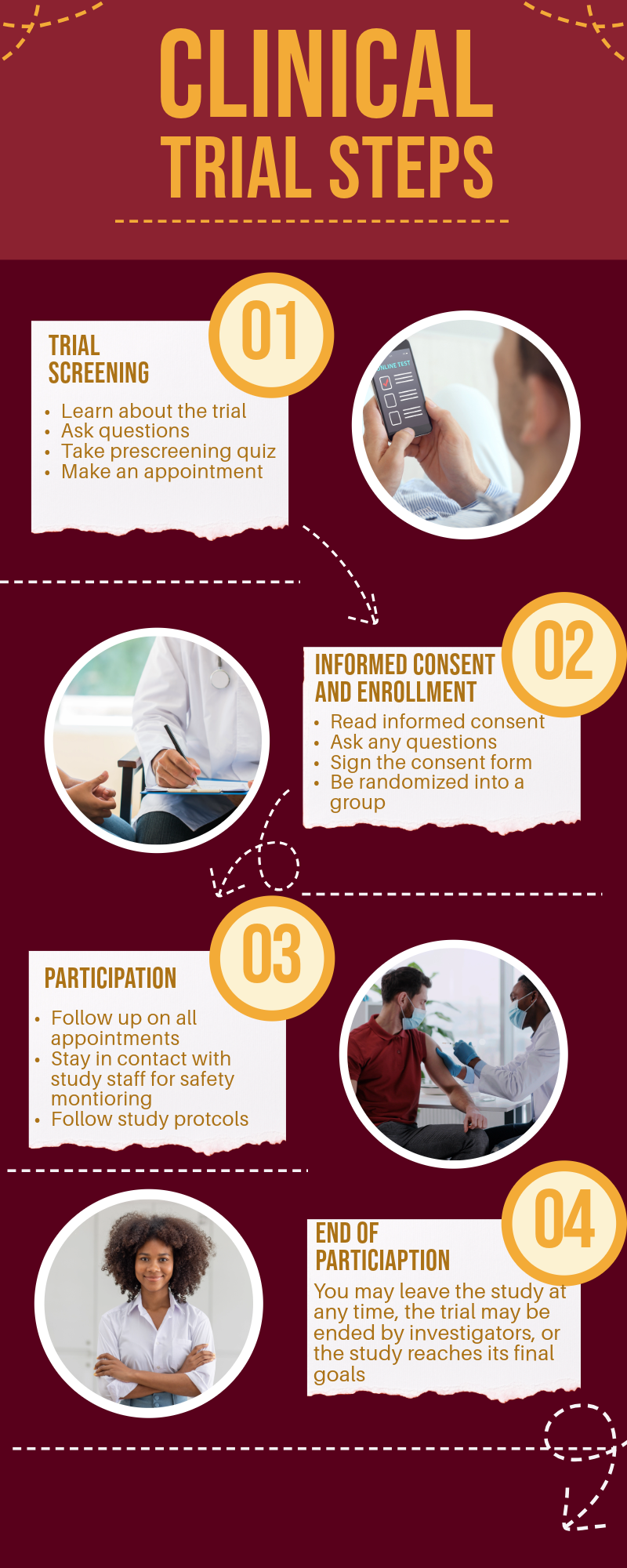
Prescreening and Questions
Before deciding to join a study, we encourage you to gather as much information as possible about the trial. Details about each clinical trial are available online, and you can reach out to the Clinical Research Coordinator (CRC) by email or leave your contact information on our website.
If you’re interested, you can take a prescreening assessment linked under each actively enrolling trial. This will help determine if you might be eligible to participate. You’re also welcome to schedule an in-person appointment at our clinical office to discuss the trial with the research team, review eligibility criteria, and learn more about the trial’s purpose, potential benefits, risks, and the time commitment required.
Informed Consent and Enrollment
If you qualify and are interested in moving forward, you can schedule an appointment to review and sign the informed consent document. This document will explain the study’s purpose, procedures, and locations, as well as any potential risks and benefits of participation. It will also outline alternatives to participation, compensation details, confidentiality measures, and your rights as a participant.
The Clinical Research Coordinator will walk you through the document and answer any questions you have. If you choose to participate, you’ll sign the document, and your enrollment in the study will begin. After that, you will be randomly assigned to a study group for the remainder of the trial.
Participation
At the start of the study, baseline measurements will be taken, which may include blood work, vitals, body measurements, and questionnaires. The research team will provide clear instructions on what’s expected, including how often you’ll need to visit the trial site and any at-home tasks you need to complete.
Depending on the trial, you may participate in phone check-ins, in-person visits, questionnaires, osteopathic manipulative treatment (OMT) or sham treatments, exercises, and blood sample collection. Throughout the trial, your safety is our top priority, and you are free to withdraw from the study at any point without any obligation.
End of Trial Participation
When your participation ends—whether you’ve completed the trial, it concludes early, or you decide to withdraw—you will no longer have any obligations. After the trial ends, the research team may follow up for additional information if agreed upon in advance. You can also access the trial’s results or contact the research team for updates on the findings.
The Clinical Trial Process